
Our Products & Technologies
Using our sophisticated technologies for assessing DOAC in urine we create unique products, providing meaningful data to health care professionals, all in an easy, rapid assessment process
The DOAC Dipstick Product
The DOAC Dipstick (TM) is the first point-of care testing system which can detect reliably and accurately whether a patient has taken Direct Oral Anti-Coagulants (DOAC), e.g. in critical care settings where every minute counts.
The diagnostic test strip DOAC Dipstick is intended for qualitative detection of the absence or presence of direct oral anticoagulants (DOACs: Dabigatran, Apixaban, Edoxaban, and Rivaroxaban) in human urine by visual identification of colours. The DOAC Dipstick is an in vitro diagnostic test and can be used at the Point of Care (POCT / Near Patient Test) or in the laboratory.
The DOAC Dipstick is intended for professional use only
It received certification under the IVDR (EU) 2017/746 regulation, has the CE Mark, is registered with the Australian TGA and with UK's MHRA, and received ANVISA approval in Brazil.
One DOAC Dipstick pack includes 12 tests (test strips).

By its rapid, qualitative point-of-care testing, use of the DOAC Dipstick can support medical decision-making and help avoiding uncontrolled, potentially fatal bleeding, e.g. in emergency care situations or in surgical interventions.
DOACs are excreted to a large extent into urine by glomerular filtration and a urine-based test can provide greater accuracy than a blood test, as there are no adversely interacting proteins or blood cells. Thus, for our urine-based DOAC Dipstick test a high sensitivity and specificity has been demonstrated (see below).
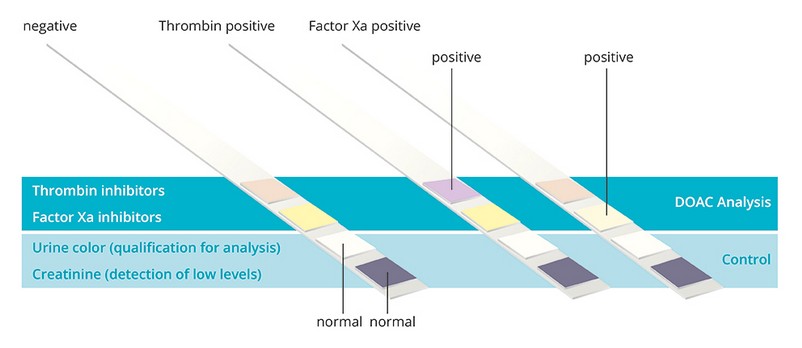
The test product allows to identify the absence or presence of:
- Direct oral Thrombin inhibitors (Dabigatran - pad #4)
- Direct oral Factor Xa inhibitors (Apixaban, Edoxaban and Rivaroxaban - pad #3)
- Urine color (for avoiding that dark urine colors impact the DOAC test results - pad #2)
- Creatinine (for detection of low creatine levels in urine - pad #1)
by means of color changes based on a chemical reaction.
Results are ready after 10 min and are obtained by color identification with the naked eye or with the DOASENSE Reader:
The DOAC Dipstick can contribute important data in many situations when it is essential to be aware of the presence or absence of DOACs for further medical decision making and therapy planning, e.g.:
- Ischemic and haemorrhagic stroke
- Major trauma
- Emergency procedures
- Spontaneous thrombotic and bleeding events
- Acute surgery or invasive interventions
- Indication for thrombolytic therapy
- Known of suspicion of deterioration of renal function
- Very elderly patients
- Adherence to therapy
- Unknown medical history
For purchase inquiries please contact our distribution partner for your respective country.
Please note that DOASENSE products are for health care professional use only!
Watch how the DOAC Dipstick works:
Accessories
Specially developed accessories complete the first suite of CE-marked products for rapid point-of-care DOAC testing:
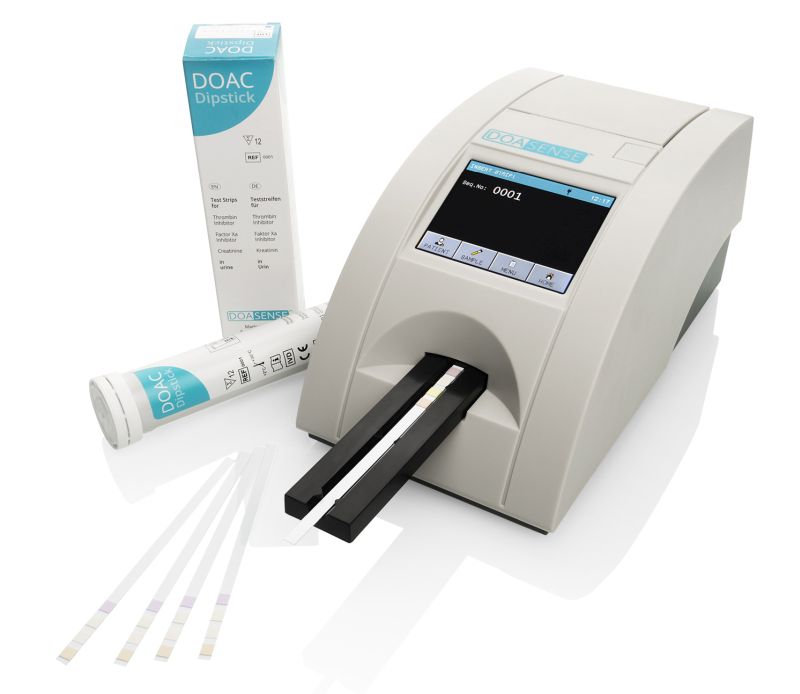
Objective documentation of results can be achieved with the compact, semi-automatic DOASENSE Reader.
The DOASENSE Reader complies with the IVDR (EU) 2017/746 regulation.
The DOASENSE Control Urines are specially designed reference substances to support your quality assurance needs.

Note: DOASENSE products are for health care professional use only!
Listen to what opinion leaders think about DOASENSE's solutions: (in German)

Our Data
Data on the DOAC Dipstick
- Harenberg J, Beyer-Westendorf J, Crowther M, Douxfils J, Elalamy I, Verhamme P, Bauersachs R, Hetjens S, Weiss C:Accuracy of a Rapid Diagnostic Test for the Presence of Direct Oral Factor Xa or Thrombin Inhibitors in Urine — A Multicenter TrialThromb Haemost 2020; 120(01): 132-140

Our Intellectual Property
DOASENSE has compiled and owns a broad portfolio of international intellectual property for protecting its proprietary approaches to DOAC testing.

NOTE: DOASENSE(TM) products may not be available or approved in your country.
* IVDR CE: DOAC Dipstick, DOASENSE Reader / IVDD CE: DOASENSE Control Urines


